Two commercially important culinary mushrooms; Pleurotus spp. and Lentinus spp. and their cultivation potential on lignocellulosic waste from aromatic plants
Review Articles | Published: 12 July, 2022
First Page: 52
Last Page: 61
Views: 4626
Keywords: Lignocellulosic biomass, Lentinus mushroom, Pleurotus mushroom, Solid-state fermentation, Aromatic plants
Abstract
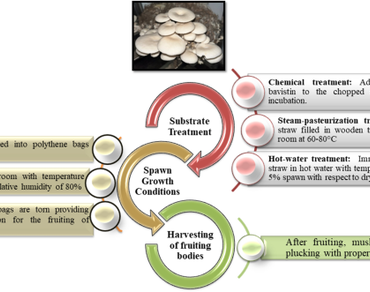
Mushrooms are considered as future vegetables as they have remarkable nutritional and medicinal properties and thus, their demand has been increasing day by day. Nowadays, mushrooms are among popular foods because of their low calorie, carbohydrates, fat and sodium content. Additionally, they are known to enhance the immune system and act as cholesterol lowering agents. Besides that, among edible mushrooms, Agaricus bisporus, Auricularia spp., Flammulina velutipes, Pleurotus spp., Lentinula edodes and Volvariella volvacea are widely cultivated around the world. Amongst these, substrate preparation for Pleurotus spp. and Lentinus spp. is relatively less gruelling, as lignocellulosic raw materials on which they are grown are abundant in nature. Cultivating these edible mushrooms through solid-state fermentation is one such eco-friendly method, which adds value to the process by utilizing agro-wastes. Therefore, various agricultural by-products have been used, as substrates for the cultivation of Pleurotus and Lentinus mushrooms. Hence, utilization of different types of lignocellulosic biomass, which are not explored yet for instance, distillation wastes of aromatic plants left after extraction of essential oils, might be a new method of mushroom cultivation and may also increase their already established medicinal and nutritional properties. Therefore, the present review focuses on medicinal and nutritional profiles of Pleurotus and Lentinus mushrooms, and is the first one of its kind to summarise the advantages of distillation waste of aromatic plants as a source of raw material for the cultivation of edible mushrooms.
References
Arunachalam K, Sasidharan SP, Yang X (2022) A concise review of mushrooms antiviral and immunomodulatory properties that may combat against COVID-19. Food Chem. https://doi.org/10.1016/j.focha.2022.100023
Atila F (2019a) Compositional changes in lignocellulosic content of some agro-wastes during the production cycle of shiitake mushroom. Sci Hort 245:263–268. https://doi.org/10.1016/j.scienta.2018.10.029
Atila F (2019b) The use of phenolic-rich agricultural wastes for Hericium erinaceus and Lentinula edodes cultivation. Ege Üniv Ziraat Fak Derg 56:417–425. https://doi.org/10.20289/zfdergi.528957
Atila F (2021) Cultivation and utilization of shiitake mushroom. In: Ekiert HM, Ramawat KG, Arora J (eds) Medicinal plants. Sustainable development and biodiversity, 28th edn. Springer, Cham. https://doi.org/10.1007/978-3-030-74779-4_13
Barros L, Baptista P, Correia DM, Casal S, Oliveira B, Ferreira IC (2007) Fatty acid and sugar compositions, and nutritional value of five wild edible mushrooms from Northeast Portugal. Food Chem 105:140–145. https://doi.org/10.1016/j.foodchem.2007.03.052
Barros L, Correia DM, Ferreira IC, Baptista P, Santos-Buelga C (2008) Optimization of the determination of tocopherols in Agaricus sp. edible mushrooms by a normal phase liquid chromatographic method. Food Chem 110:1046–1050. https://doi.org/10.1016/j.foodchem.2008.03.016
Bernardi E, Minotto E, Nascimento JSD (2013) Evaluation of growth and production of Pleurotus sp. in sterilized substrates. Arq Inst Biol 80:318–324
Bisen PS, Baghel RK, Sanodiya BS, Thakur GS, Prasad GBKS (2010) Lentinus edodes: a macrofungus with pharmacological activities. Curr Med Chem 17:2419–2430. https://doi.org/10.2174/092986710791698495
Chopra H, Mishra A, Baig A, Mohanta T, Mohanta Y, Baek K (2021) Narrative review: bioactive potential of various mushrooms as the treasure of versatile therapeutic natural product. J Fungi 7:728. https://doi.org/10.3390/jof7090728
Curvetto N, Figlas D, Delmastro S (2002) Sunflower seed hulls as substrate for the cultivation of shiitake mushrooms. Hort Technol 12:652–655
Debayo EA, Martınez-Carrera D (2015) Oyster mushrooms (Pleurotus) are useful for utilizing lignocellulosic biomass. Afr J Biotechnol 14(1):52–67. https://doi.org/10.5897/AJB2014.14249
Deka H, Deka S, Baruah CK, Das J, Hoque S, Sarma H, Sarma NS (2011) Vermicomposting potentiality of Perionyx excavatus for recycling of waste biomass of java citronella-an aromatic oil yielding plant. Bioresour Technol 102:11212–11217. https://doi.org/10.1016/j.biortech.2011.09.102
Dicks L, Ellinger S (2020) Effect of the intake of oyster mushrooms (Pleurotus ostreatus) on cardiometabolic parameters-a systematic review of clinical trials. Nutr J 12:1134. https://doi.org/10.3390/nu12041134
Dulay RMR, Garcia EJB (2017) Optimization and enriched cultivation of Philippine (CLSU) strain of Lentinus strigosus (BIL1324). Biocatal Agric Biotechnol 12:323–328. https://doi.org/10.1016/j.bcab.2017.10.023
Dutt P, Mitra D, Ranjan P (2009) International trade and unemployment: theory and cross-national evidence. J Int Econ 78:32–44. https://doi.org/10.1016/j.jinteco.2009.02.005
Feeney M, Dwyer J, Hasler-Lewis C, Milner J, Noakes M, Rowe S (2014) Mushrooms and health summit proceedings. Nutr J 144:1128S-1136S. https://doi.org/10.3945/jn.114.190728
Ferreira IC, Barros L, Abreu R (2009) Antioxidants in wild mushrooms. Curr Med Chem 16:1543–1560
Gaitán-Hernández R, Norberto Cortés G (2014) Improvement of yield of the edible and medicinal mushroom Lentinula edodes on wheat straw by use of supplemented spawn. Braz J Microbiol 45:467–474. https://doi.org/10.1590/s1517-83822014000200013
Geng H, Yuan Z, Fan Q, Dai X, Zhao Y, Wang Z, Qin M (2014) Characterisation of cellulose films regenerated from acetone/water coagulants. Carbohydr Polym 102:438–444. https://doi.org/10.1016/j.carbpol.2013.11.071
George PL, Ranatunga TD, Reddy SS, Sharma GC (2014) A comparative analysis of mineral elements in the mycelia and the fruitbodies of shiitake mushrooms. Am J Food Tech 9:360–369
Girmay Z, Gorems W, Birhanu G et al (2016) Growth and yield performance of Pleurotus ostreatus (Jacq. Fr.) Kumm (oyster mushroom) on different substrates. AMB Express 6(1):87
Golak-Siwulska I, Kaluzewicz A, Spizewski T (2018) Bioactive compounds and medicinal properties of Oyster mushrooms (Pleurotus sp.). Folia Hortic 30:191–201
Gonzalez A, Cruz M, Losoya C, Nobre C, Loredo A, Rodriguez R, Belmares R et al (2020) Edible mushrooms as a novel protein source for functional foods. Food Funct 11:7400–7414. https://doi.org/10.1039/D0FO01746A
Gregory A, Pohleven F (2014) Cultivation of three medicinal mushroom species (Ganoderma lucidum, Lentinula edodes and Grifola frondosa) on olive oil press cakes containing substrates and their bioactivity assessment. Ann Agrar Sci 12(3):35–38
Guo Z, Hu Y, Wang D, Ma X, Zhao X, Zhao B, Wang J, Liu P (2009) Sulfated modification can enhance the adjuvanticity of lentinan and improve the immune effect of ND vaccine. Vaccine 27:660–665. https://doi.org/10.1016/j.vaccine.2008.11.038
Heleno SA, Barros L, Sousa MJ, Martins A, Ferreira IC (2010) Tocopherol’s composition of Portuguese wild mushrooms with antioxidant capacity. Food Chem 119:1443–1450. https://doi.org/10.1016/j.foodchem.2009.09.025
Hernandez RG, López-Peña D, Esqueda M, Gutiérrez A (2019) Review of bioactive molecules production, biomass, and basidiomata of shittake culinary-medicinal mushrooms, Lentinus edodes (Agaricomycetes). Int J Med Mushrooms 21:841–850. https://doi.org/10.1615/IntJMedMushrooms.2019031849
Hibbett DS (2001) Shiitake mushrooms and molecular clocks: historical biogeography of Lentinula. J Biogeog 28:231–241. https://doi.org/10.1046/j.1365-2699.2001.00528.x
Hoa HT, Wang CL (2015) The effects of temperature and nutritional conditions on mycelium growth of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 43:14–23. https://doi.org/10.5941/MYCO.2015.43.1.14
Hsu CM, Hameed K, Cotter V et al (2018) Isolation of mother cultures and preparation of spawn for oyster mushroom cultivation. Univ Florida IFAS Extension EDIS series, Gainesville
Irshad M, Subhani MA, Ali S, Hussain A (2020) Biological importance of essential oils. Am J Essent Oil Nat Prod. https://doi.org/10.5772/intechopen.87198
Jang KY, Oh YL, Oh M et al (2016) Introduction of the representative mushroom cultivars and ground-breaking cultivation techniques in Korea. J Mushroom 14:136–141. https://doi.org/10.14480/JM.2016.14.4.136
Jasinghe VJ, Perera CO (2006) Ultraviolet irradiation: the generator of vitamin D2 in edible mushrooms. Food Chem 95:638–643. https://doi.org/10.1016/j.foodchem.2005.01.046
Jatwa TK, Apet KT, Wagh SS et al (2016) Evaluation of various agro-wastes for production of Pleurotus spp. (P. florida, P. sajor-caju and P. eous). J Pure Appl Microbiol 10:2783–2792. https://doi.org/10.22207/JPAM.10.4.37
Jegadeesh R, Lakshmanan H, Kab-Yeul J et al (2018) Cultivation of pink oyster mushroom Pleurotus djamor var. roseus on various agro-residues by low-cost technique. J Mycopathol Res 56:213–220
Jensen MM, Djajadi DT, Torri C, Rasmussen HB, Madsen RB, Venturini E, Glasius M et al (2018) Hydrothermal liquefaction of enzymatic hydrolysis lignin: biomass pretreatment severity affects lignin valorization. ACS Sustain Chem Eng 6:5940–5949. https://doi.org/10.1021/acssuschemeng.7b04338
Kakon AJ, Md Choudhury BK, Shusmita S (2012) Mushroom is an ideal food supplement. J Dhaka Natl Med Coll Hosp 18:58–62. https://doi.org/10.3329/jdnmch.v18i1.12243
Kalauni D, Joshi A (2018) Status of medicinal and aromatic plant (maps) and socio-economic influence in Nepalese livelihood-a review research. Acta Sci Agric 2(9):123–130
Karneva K, Vasileva I, Denev P, Denkova R, Shikov V, Manolova M et al (2018) Valorization of lavender waste: obtaining and characteristics of polyphenol rich extracts. Food Sci Biotechnol 1:11. https://doi.org/10.30721/fsab2018.v1.i1.5
Knop D, Yarden O, Hadar Y (2015) The ligninolytic peroxidases in the genus Pleurotus: divergence in activities, expression, and potential applications. Appl Microbiol Biotechnol 99(3):1025–1038
Kumar K (2020) Nutraceutical potential and processing aspects of oyster mushrooms (Pleurotus species). Curr Nutr Food Sci 16:3–14. https://doi.org/10.2174/1573401314666181015111724
Kumari K (2020) Mushrooms as source of dietary fiber and its medicinal value: a review article. J Pharmacogn Phytochem 9:2075–2078
Kumla J, Suwannarach N, Sujarit K, Penkhrue W, Kakumyan P, Jatuwong K et al (2020) Cultivation of mushrooms and their lignocellulolytic enzyme production through the utilization of agro-industrial waste. Molecules 25:2811. https://doi.org/10.3390/molecules25122811
Lavelli V, Proserpio C, Gallotti F et al (2018) Circular reuse of bio-resources: the role of Pleurotus spp. in the development of functional foods. Food Funct 9:1353–1372. https://doi.org/10.1039/C7FO01747B
Lee SJ, Kim HH, Kim SH et al (2018) Culture conditions of liquid spawn and the growth characteristics of Pleurotus ostreatus. J Mushrooms 16:162–170. https://doi.org/10.14480/JM.2018.16.3.162
Lesage-Meessen L, Bou M, Sigoillot J, Faulds C, Lomascolo A (2015) Essential oils and distilled straws of lavender and lavandin: a review of current use and potential application in white biotechnology. Appl Microbiol Biotechnol 99:3375–3385. https://doi.org/10.1007/s00253-015-6511-7
Levanon D, Rothschild N, Danai O, Masaphy S (1993) Bulk treatment of substrate for the cultivation of shiitake mushrooms (Lentinus edodes) on straw. Bioresource Technol 45:63–64. https://doi.org/10.1016/0960-8524(93)90145-2
Liu B, Bau YS (1980) Fungi pharmacopoeia. Kinoko, Oakland, CA. A translation of Chinese medicinal fungi, 2nd edn. Shansi People Publishing House, Shansi, p 302
Maiti K, Mukherjee K, Gantait A, Saha B, Mukherjee P (2007) Curcumin–phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm 330:155–163. https://doi.org/10.1016/j.ijpharm.2006.09.025
Mamimin C, Chanthong S, Leamdum C, Sompong O, Prasertsan P (2021) Improvement of empty palm fruit bunches biodegradability and biogas production by integrating the straw mushroom cultivation as a pretreatment in the solid-state anaerobic digestion. Bioresour Technol 319:124227. https://doi.org/10.1016/j.biortech.2020.124227
Mata JL, Petersen RH (2000) A new species of Lentinula (Agaricales) from Central America. Mycoscience 41:351–355. https://doi.org/10.1007/BF02463948
Mata JL, Petersen RH, Hughes KW (2001) The genus Lentinula in the Americas. Mycologia 93:1102–1112. https://doi.org/10.1080/00275514.2001.12063244
Mattila P, Konko K, Eurola M, Pihlava JM, Astola J, Vahteristo L, Piironen V (2001) Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J Agric Food Chem 49:2343–2348. https://doi.org/10.1021/jf001525d
Mattila P, Salo-Vaananen P, Konko K, Aro H, Jalava T (2002) Basic composition and amino acid contents of mushrooms cultivated in Finland. J Agric Food Chem 50:6419–6422. https://doi.org/10.1021/jf020608m
Moonmoon M, Shelly NJ, Khan MA, Uddin MN, Hossain K, Tania M, Ahmed S (2011) Effects of different levels of wheat bran, rice bran and maize powder supplementation with saw dust on the production of shiitake mushroom (Lentinus edodes (Berk.) Singer). Saudi J Biol Sci 18:323–328. https://doi.org/10.1016/j.sjbs.2010.12.008
Mowsurni F, Chowdhury M (2013) Oyster mushroom: biochemical and medicinal prospects. Bangladesh J Med Biochem 3:23–28. https://doi.org/10.3329/bjmb.v3i1.13804
Munda S, Begum T, Gogoi A, Pandey SK, Sarma N, Lal M (2022) Induced variations by gamma radiation and EMS on the agronomic traits, essential oil yield with its quality and their exploitation in Java citronella (Cymbopogon winterianus Jowitt). Int J Radiat Biol. https://doi.org/10.1080/09553002.2022.2038805
Naraian R, Sahu RK, Kumar S et al (2009) Influence of different nitrogen rich supplements during cultivation of Pleurotus florida on corn cob substrate. Environmentalist 29:1–7. https://doi.org/10.1007/s10669-008-9174-4
Netherway T, Bahram M (2021) Fungal Biogeography. Biogeography: an integrative approach of the evolution of living. Wiley, New Jersey, pp 193–218
Okolo KO, Siminialayi IM, Orisakwe OE (2016) Protective effects of Pleurotus tuber-regium on carbon-tetrachloride induced testicular injury in Sprague Dawley rats. Front Pharmacol 7:1–6. https://doi.org/10.3389/fphar.2016.00480
Okwulehie IC, Nosike EN (2015) Phytochemicals and vitamin compositions of Pleurotus pulmonarius cultivated on barks of some indigenous fruit trees supplemented with agro-wastes. Asian J Plant Sci 5:1–7
Oyetayo VO, Ogidi CO, Bayode SO, Enikanselu FF (2021) Evaluation of biological efficiency, nutrient contents and antioxidant activity of Pleurotus pulmonarius enriched with Zinc and Iron. Indian Phytopathol 74:901–910. https://doi.org/10.1007/s42360-021-00410-7
Pandey AK, Kumar P, Saxena MJ, Maurya P (2020) Distribution of aromatic plants in the world and their properties. Feed additives. Academic Press, London, pp 89–114
Patil SS, Ahmed SA, Telang SM et al (2010) The nutritional value of Pleurotus ostreatus (jacq: fr.) Kumm cultivated on different lignocellulosic agrowastes. Innov Rom Food Biotechnol 7:66–76
Pereira E, Barros L, Martins A, Ferreira IC (2012) Towards chemical and nutritional inventory of Portuguese wild edible mushrooms in different habitats. Food Chem 130:394–403. https://doi.org/10.1016/j.foodchem.2011.07.057
Phonemany M, Raghoonundon B, Luangharn T, Tang SM, Hyde KD, Thongklang N (2021) A mini review on the potential pharmacological properties, cultivation, and market value of edible Lentinus mushrooms (Polyporaceae). Fungal Biotec 1:49–64. https://doi.org/10.5943/funbiotec/1/2/4
Piazza S, Benvenuti M, Damonte G, Cecchi G, Mariotti MG, Zotti M (2021) Fungi and circular economy: Pleurotus ostreatus grown on a Substrate with agricultural waste of lavender, and its promising biochemical profile. Recycling 6:40. https://doi.org/10.3390/recycling6020040
Pylak M, Oszust K, Frąc M (2019) Review report on the role of bioproducts, biopreparations, biostimulants and microbial inoculants in organic production of fruit. Rev Environ Sci Biotechnol 18:597–616. https://doi.org/10.1007/s11157-019-09500-5
Raman J, Nanjian R, Lakshmanan H et al (2014) Hypolipidemic effect of Pleurotus djamor var. roseus in experimentally induced hypercholesteromic rats. Res J Pharm Biol Chem Sci 5:581–588. https://doi.org/10.1016/j.procbio.2014.11.003
Raman J, Lee SK, Im JH, Oh MJ, Oh YL, Jang KY (2018) Current prospects of mushroom production and industrial growth in India. J Mushrooms 16:239–249. https://doi.org/10.14480/JM.2018.16.4.239
Raman J, Jang KY, Oh YL, OhM IJH, Lakshmanan H, Sabaratnam V (2021) Cultivation and nutritional value of prominent Pleurotus spp.: an overview. Mycobiology 49:1–14. https://doi.org/10.1080/12298093.2020.1835142
Reis FS, Barros L, Martins A, Ferreira IC (2012) Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: an inter-species comparative study. Food Chem Toxicol 50:191–197. https://doi.org/10.1016/j.fct.2011.10.056
Rossi IH, Monteiro AC, Machado JO, Andrioli JL, Barbosa JC (2003) Shiitake (Lentinula edodes) production on a sterilized bagasse substrate enriched with rice bran and sugarcane molasses. Braz J Microbiol 34:66–71. https://doi.org/10.1590/S1517-83822003000100014
Royse DJ (1996) Yield stimulation of shiitake by millet supplementation of wood chip substrate. In: Royse DJ (ed) Mushroom biology and mushroom products. University Park, Penn State University Press, College Station, pp 277–283
Royse DJ, Sanchez JE (2007) Ground wheat straw as a substitute for portions of oak wood chips used in shiitake (Lentinula edodes) substrate formulae. Bioresource Technol 98:2137–2141. https://doi.org/10.1016/j.biortech.2006.08.023
Royse DJ (2014) A global perspective on the high five: Agaricus, Pleurotus, Lentinula, Auricularia & Flammulina. Paper presented at: The 8th International Conference on Mushroom Biology and Mushroom Products. November 19–22; New Delhi, India.
Salo K, Domisch T, Kouki J (2019) Forest wildfire and 12 years of post-disturbance succession of saprotrophic macrofungi (Basidiomycota, Ascomycota). For Ecol Manag 451:11454. https://doi.org/10.1016/j.foreco.2019.117454
Sardar H, Ali MA, Anjum MA et al (2017) Agro-indus- trial residues influence mineral elements accumulation and nutritional composition of king oyster mushroom (Pleurotus eryngii). Sci Hortic 225:327–334. https://doi.org/10.1016/j.scienta.2017.07.010
Schneider I, Kressel G, Meyer A et al (2011) Lipid lowering effects of oyster mushroom (Pleurotus ostreatus) in humans. J Funct Foods 3:17–24. https://doi.org/10.1016/j.jff.2010.11.004
Singh MP, Rai SN, Dubey SK, Pandey AT, Tabassum N, Chaturvedi VK, Singh NB (2021) Biomolecules of mushroom: a recipe of human wellness. Crit Rev Biotechnol. https://doi.org/10.1080/07388551.2021.1964431
Singh MP, Singh VK (2011) Yield performance and nutritional analysis of Pleurotus citrinopileatus on different agro wastes and vegetable wastes. Paper presented at: The 7th International Conference on Mushroom Biology and Mushroom Products October 4–7; Arcachon, France.
Smigielski K, Prusinowska R, Stobiecka A, Kunicka-Styczyñska A, Gruska R (2018) Biological properties and chemical composition of essential oils from flowers and aerial parts of lavender (Lavandula angustifolia). J Essent 21:1303–1314. https://doi.org/10.1080/0972060X.2018.1503068
Stamets P (2000) Growing gourmet and medicinal mushrooms. TenSpeed Press, Berkeley, p 150
Szczerbowski D, Pitarelo A, Zandoná Filho A, Ramos L (2014) Sugarcane biomass for biorefineries: comparative composition of carbohydrate and non-carbohydrate components of bagasse and straw. Carbohydr Polym 114:95–101. https://doi.org/10.1016/j.carbpol.2014.07.052
Tang C, Hoo PC, Tan LT et al (2016) Golden needle mushroom: a culinary medicine with evidenced based biological activities and health promoting properties. Front Pharmacol 7:474. https://doi.org/10.3389/fphar.2016.00474
The State of the World's Plants report 2017, Kew. Kew.org (2022) https://www.kew.org/about-us/press-media/state-of-the-worlds-plants-2017. Retrieved 15 Apr 2022
Truzzi E, Chaouch M, Rossi G, Tagliazucchi L, Bertelli D, Benvenuti S (2022) Characterization and valorization of the agricultural waste obtained from Lavandula steam distillation for its reuse in the food and pharmaceutical fields. Molecules 27:1613. https://doi.org/10.3390/molecules27051613
Turło J, Gutkowska B, Herold F, Krzyczkowski W, Błażewicz A, Kocjan R (2008) Optimizing vitamin B12 biosynthesis by mycelial cultures of Lentinula edodes (Berk.) Pegl. Enzyme Microb Technol 43:369–374
Turrini F, Zunin P, Boggia R (2021) Potentialities of rapid analytical strategies for the identification of the botanical species of several “specialty” or “gourmet” oils. Foods 10:183. https://doi.org/10.3390/foods10010183
Valverde M, Hernández-Pérez T, Paredes-López O (2015) Edible mushrooms: improving human health and promoting quality life. Int J Microbiol. https://doi.org/10.1155/2015/376387
Xiao G, Zhang M, Shan L et al (2011) Extension of the shelf-life of fresh oyster mushrooms (Pleurotus ostreatus) by modified atmosphere packaging with chemical treatments. Afr J Bitechnol 10:9509–9517. https://doi.org/10.5897/AJB08.974
Yang W, Guo F, Wan Z (2013) Yield and size of oyster mushroom grown on rice/wheat straw basal substrate supplemented with cotton seed hull. Saudi J Biol Sci 20:333–338. https://doi.org/10.1016/j.sjbs.2013.02.006
Yehia RS (2012) Nutritional value and biomass yield of the edible Mushroom Pleurotus ostreatus cultivated on different wastes in Egypt. Innov Rom Food Biotechnol 11:9–14
Yohalem D, Passey T (2011) Amendment of soils with fresh and post-extraction lavender (Lavandula angustifolia) and lavandin (Lavandula intermedia) reduce inoculum of Verticillium dahliae and inhibit wilt in strawberry. Appl Soil Ecol 49:187–196. https://doi.org/10.1016/j.apsoil.2011.05.006
Zamakshshari NH, Ahmed IA, Didik NAM, Nasharuddin MNA, Hashim NM, Abdullah R (2022) Chemical profile and antimicrobial activity of essential oil and methanol extract from peels of four Durio zibethinus L. varieties. Biomass Convers. https://doi.org/10.1007/s13399-021-02134-0
Zervakis G, Koutrotsios G (2017) Solid-state fermentation of plant residues and agro-industrial wastes for the production of medicinal mushrooms. Med Aromat Plants World. https://doi.org/10.1007/978-981-10-5978-0_12
Author Information
CSIR-Indian Institute of Integrative Medicine, Jammu, India